The LumiCycle apparatus fits inside a standard incubator. An internal fan circulates the incubator air to maintain the proper temperature within the chamber. The temperature is therefore as stable as the incubator can make it. The turntable and photon counting are fully automated. System setup and operation is straightforward. Online help files are included with the software.
Two-colour option. With the 2-colour adaptation, coloured filters can be placed on 2 of the photomultipliers so that 16 samples can be recorded simultaneously in 2 different colours. To create the image on the right, fibroblasts were transfected with Bmal-Nluc and Per2-Fluc, and recorded with a 550 nm long-pass filter on one PMT and 500 nm short-pass filter on the other. Optical filters can be easily changed by the user, or they can be removed for recording in the standard, non-colour mode.
In addition to its high-precision photon counting hardware, LumiCycle has the most flexible and easy-to-use software for the collection and analysis of circadian rhythms in luminometry data.
Features
- Start and stop each counting channel asynchronously. You may, for example, start one experiment with 10 samples on one day, and start a second experiment with 22 samples the following day, stop the first experiment 5 days later and start yet a third set of dishes in their place.
- Interrupt the data collection to perform experimental manipulations on the samples (such as adding a drug). Once the treatment is complete, LumiCycle will pick up exactly where it left off.
- Flexible counting schedules, from once per hour, to once per minute.
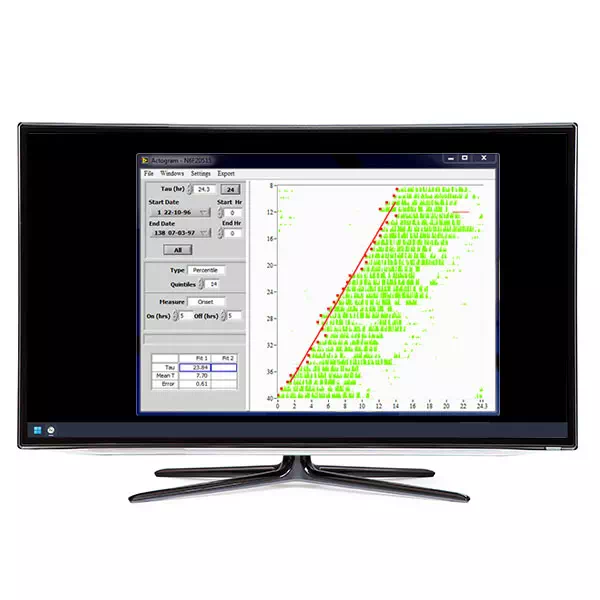
- View records during the data collection process. A single window shows data for all 32 channels at once. Double-click on one of the graphs to view the data in greater detail.
- Analysis program automatically compensates for baseline shifts and signal attenuation over the course of the trial. The dominant circadian period and phase can then be extracted. View periodograms and actograms, calculate phase shifts, extract periods with spectral or periodogram methods.
- Superimpose multiple records for comparison and figure preparation.
- Calculate rhythm parameters for the complete record, or for multiple subsets of the record. If, for example, a treatment is given halfway through a record that is expected to create a period or phase change, the two halves of the record can be analyzed separately and a phase shift calculated.
- While data collection is in progress, you may repeatedly copy the growing data files onto another computer (viaAnalysis a floppy or the network) and analyze the data collected so far for period and phase. Data collectioncontinues uninterrupted until the end of the experiment.
- Extracted periods and phases can be exported to a text file for later manipulation in Excel or other statistics programs.
- Raw or baseline-subtracted records can be exported to ClockLab for further analysis using periodograms, actograms, and activity profiles.
Specifications:
| Tissue holders | 35 mm dishes (Falcon) |
|---|---|
| Capacity | 32 dishes |
| Detectors |
4 photomultiplier tubes (photon counting mode) Each tube records alternately from 8 dishes |
| Dark counts |
< 10/s @ 20 °C 50/s (typical) @ 35 °C |
|
Optical filters for color separation |
Diameter: 25 mm Thickness: 3 mm Long Pass (from Edmund Optics) Short Pass (from Edmund Optics) |
| Operating temperature |
Up to 37 °C — ambient humidity (as set by incubator or environmental room) |
| Dimensions |
14″ W × 13.5″ H × 14″ D (35.5 × 34.3 × 35.5 cm) Front loading |
| Count period | 10 s – 1 hr (program selectable) |
| System requirements |
Windows 7/8/10 1 USB port 1280 × 1024 resolution monitor recommended |
| Analyses |
Actogram Periodogram Spectral (sinusoidal fit and damped sinusoidal fit) Phase shift (double sinusoidal fit to defined periods) Baseline subtraction (low-order polynomial) |
| Warranty | One year, parts and labor |
Scientific Resources
- Alcántara-Alonso, V., Dallmann, R., Lehnert, H., de Gortari, P., & Grammatopoulos, D. K. (2023). CRH-R2 signalling modulates feeding and circadian gene expression in hypothalamic mHypoA-2/30 neurons. Frontiers in Endocrinology, 14. https://doi.org/10.3389/FENDO.2023.1266081/FULL
- Draijer, S., Timmerman, R., Pannekeet, J., van Harten, A., Farshadi, E. A., Kemmer, J., van Gilst, D., Chaves, I., & Hoekman, M. F. M. (2023). FoxO3 Modulates Circadian Rhythms in Neural Stem Cells. International Journal of Molecular Sciences 2023, Vol. 24, Page 13662, 24(17), 13662. https://doi.org/10.3390/IJMS241713662
- Graniczkowska, K. B., Paulose, J. K., & Cassone, V. M. (2023). Circadian regulation of metabolic, cell division, and cation transport promoters in the gastrointestinal bacterium Klebsiella aerogenes. Frontiers in Microbiology, 14. https://doi.org/10.3389/FMICB.2023.1181756/FULL
- Hesketh, S. J., Sexton, C. L., Wolff, C. A., Viggars, M. R., & Esser, K. A. (2023). Early morning run-training results in enhanced endurance performance adaptations in mice. BioRxiv. https://doi.org/10.1101/2023.09.18.557933
- Katoku-Kikyo, N., Lim, S., Yuan, C., Koroth, J., Nakagawa, Y., Bradley, E. W., & Kikyo, N. (2023). The circadian regulator PER1 promotes cell reprogramming by inhibiting inflammatory signaling from macrophages. PLOS Biology, 21(12), e3002419. https://doi.org/10.1371/JOURNAL.PBIO.3002419
- Mawatari, K., Koike, N., Nohara, K., Wirianto, M., Uebanso, T., Shimohata, T., Shikishima, Y., Miura, H., Nii, Y., Burish, M. J., Yagita, K., Takahashi, A., Yoo, S. H., & Chen, Z. (2023). The Polymethoxyflavone Sudachitin Modulates the Circadian Clock and Improves Liver Physiology. Molecular Nutrition & Food Research, 67(9), 2200270. https://doi.org/10.1002/MNFR.202200270
- Ting, I. J., Psomas, A., Skene, D. J., & van der Veen, D. R. (2023). Reduced glucose concentration enhances ultradian rhythms in Pdcd5 promoter activity in vitro. Frontiers in Physiology, 14. https://doi.org/10.3389/FPHYS.2023.1244497/FULL