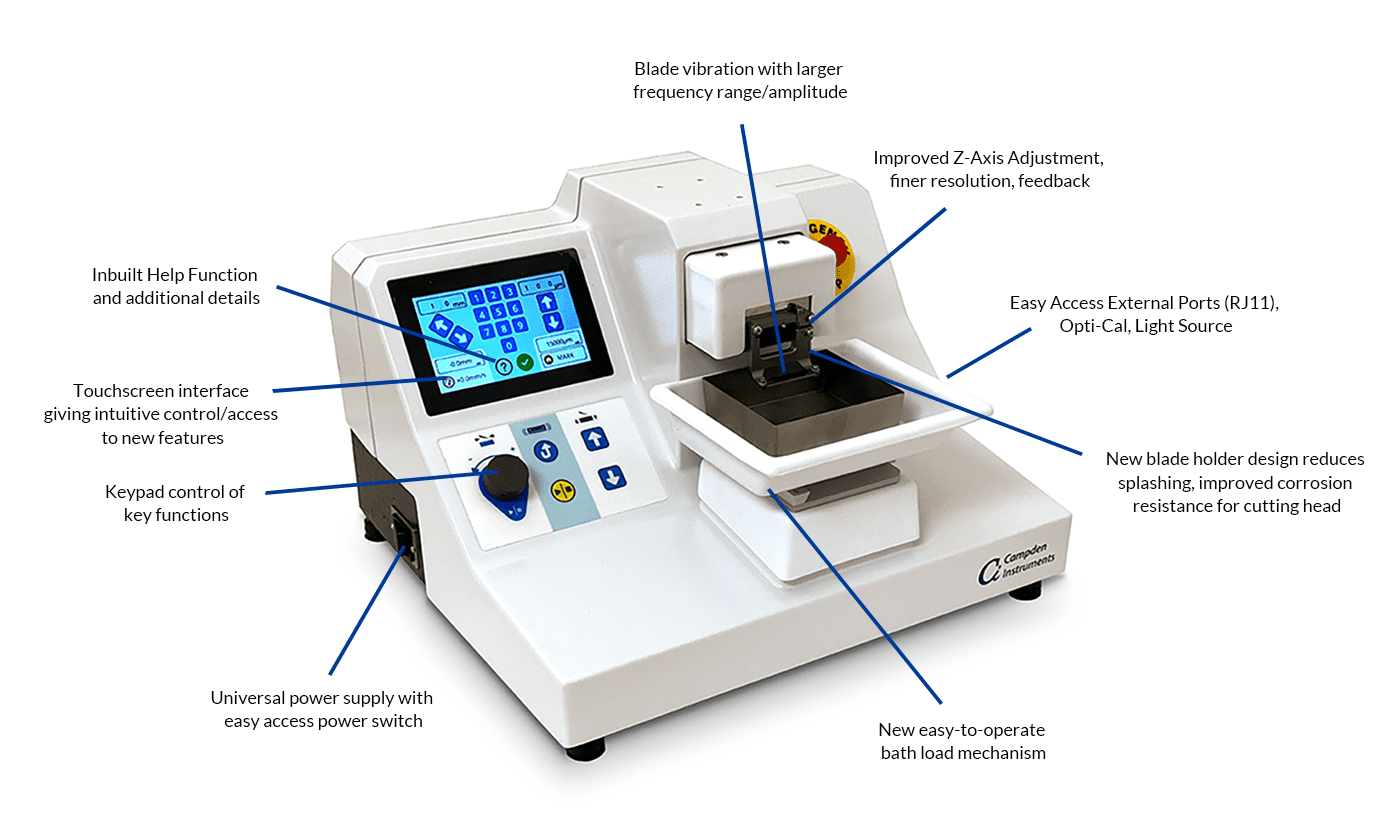
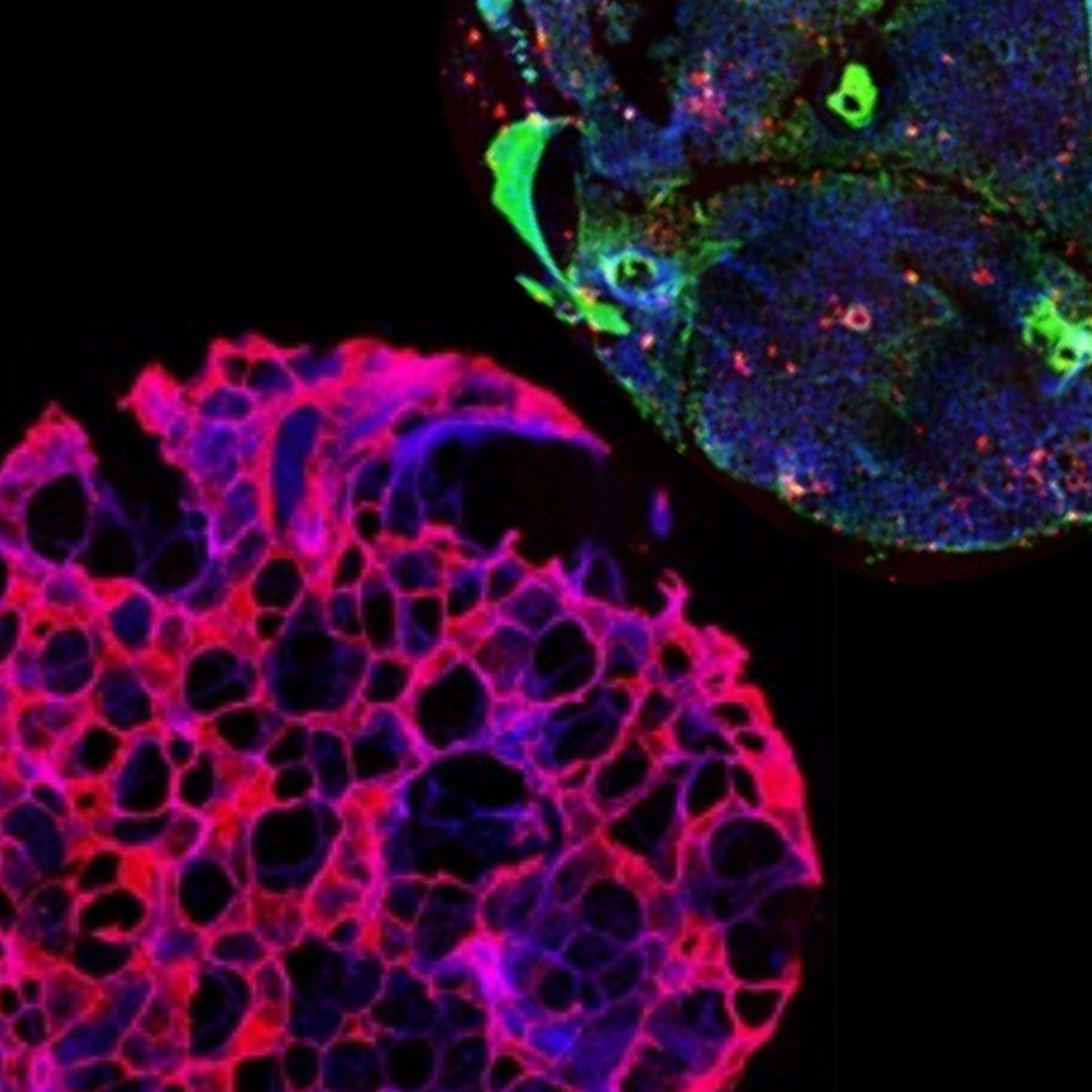
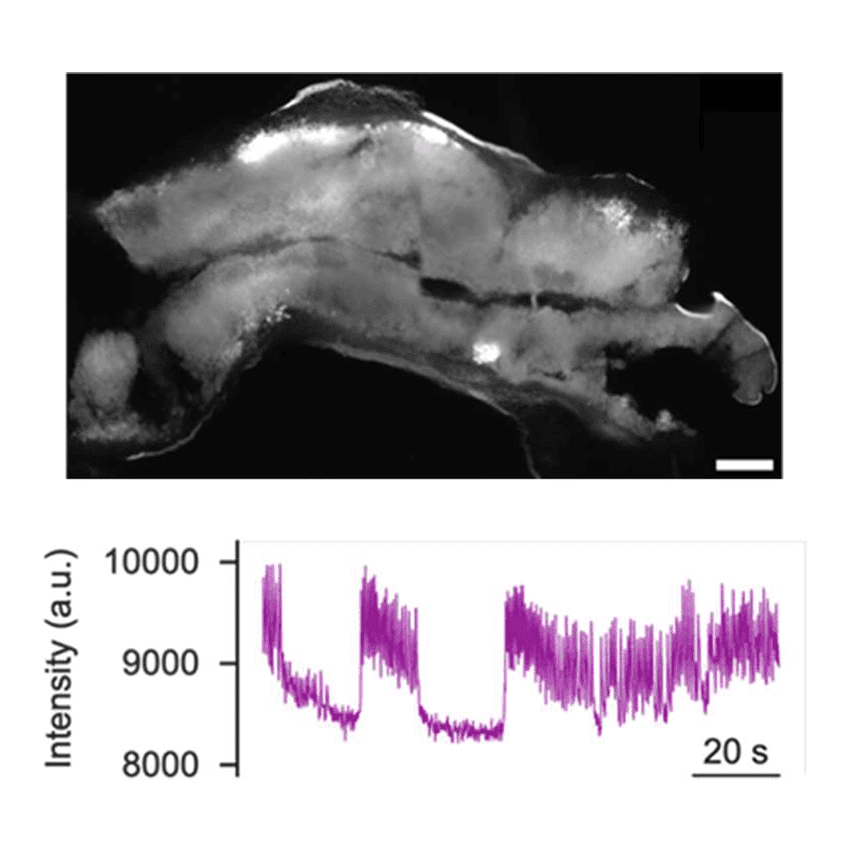
Precision-Cut Liver Slices (PCLS) are emerging as a gold-standard ex vivo model for liver research, offering a structurally and functionally intact hepatic microenvironment. PCLS preserve the multicellular architecture of the liver, including hepatocytes, Kupffer cells, hepatic stellate cells, and endothelial cells, enabling advanced studies in hepatotoxicology, metabolic diseases, liver fibrosis, and pharmacokinetics. This blog dives into the scientific principles, technical considerations, and vibratome settings essential for reproducible and high-quality liver slicing.
Why Precision-Cut Liver Slices?
- Physiological Relevance: Multicellular integrity allows for more predictive responses to stimuli.
- Species Flexibility: Applicable to human, mouse, rat, pig, and non-human primate livers.
- Ethical Viability: Reduces the number of animals required for in vivo studies.
- Experimental Versatility: Compatible with omics analyses, imaging, and functional assays.
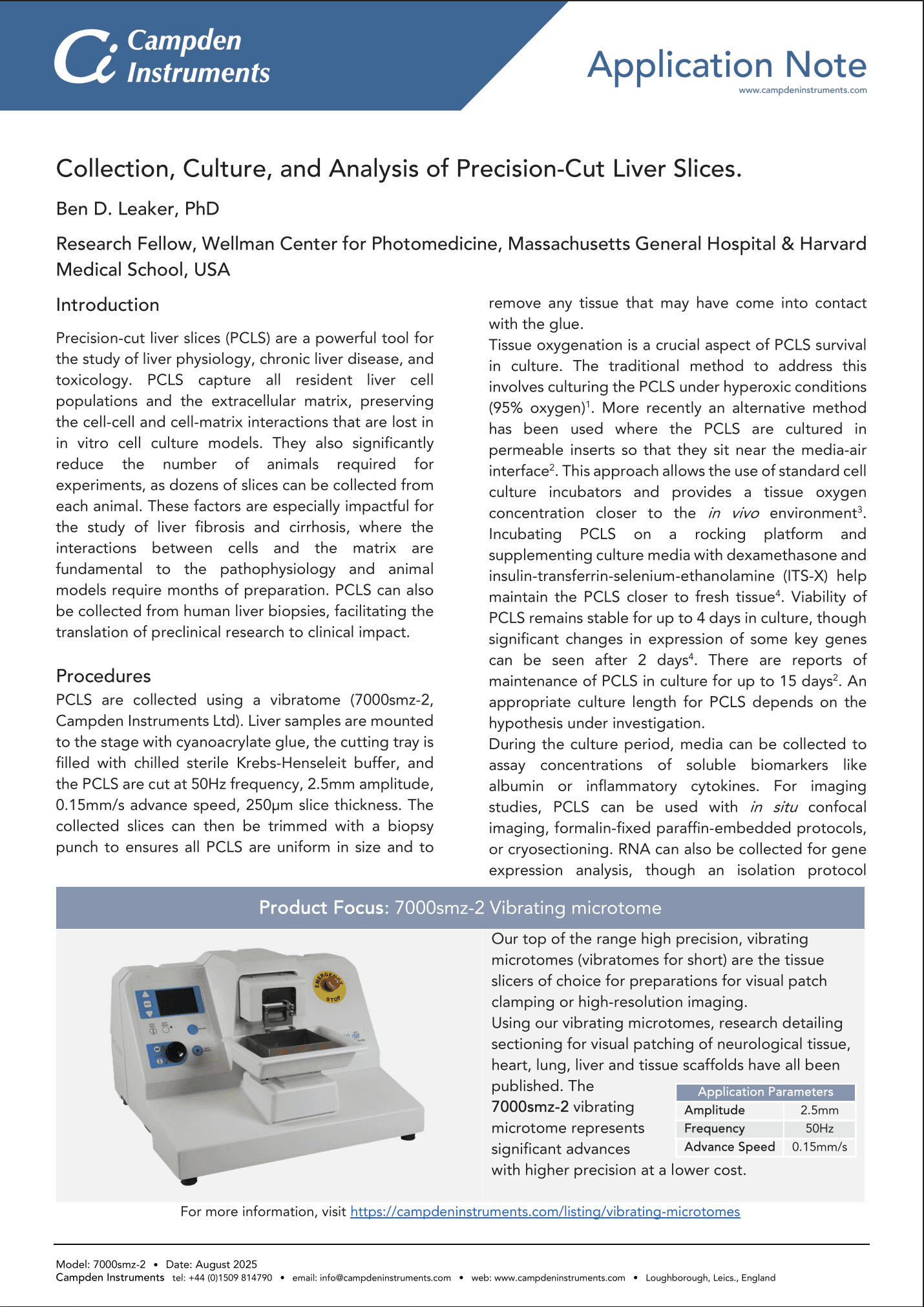
Preparation of PCLS: Vibratome Sectioning Parameters
Pre-Slicing Preparation:
- Use fresh liver tissue processed rapidly to minimize ischemic injury.
- Use a biopsy punch to produce liver columns.
- Mount colums directly on vibratome tissue mount using cyanoacrylate glue.
- Fill vibratome with cold, sterile buffer (e.g. Krebs Henseleit buffer).
Optimised Vibratome Parameters:

PCLS collection. (c) 250 µm thick PCLS were cut at 50 Hz, 2.5 mm amplitude, 0.15 mm/s
(from Leaker, B.D., Wang, Y., Tam, J. et al. Analysis of culture and RNA isolation methods for precision-cut liver slices from cirrhotic rats. Sci Rep 14, 15349 (2024). https://doi.org/10.1038/s41598-024-66235-2)
| Parameter | Recommended Setting |
|---|---|
| Slice Thickness | 200-300 µm |
| Sectioning Speed | 0.03–0.15 mm/s |
| Vibration Frequency | 50–100 Hz |
| Amplitude | 1.5–2.5 mm |
| Temperature | 4°C with cold HBSS or Williams’ E medium |
Viability and Culture Conditions
PCLS are cultured in Williams’ E medium with:
- GlutaMAX or L-glutamine
- Penicillin/Streptomycin
- Insulin-Transferrin-Selenium (ITS)
- Dexamethasone (optional)
Oxygenation: 95% O₂ / 5% CO₂ in rotating or air-liquid interface culture systems.
Viability: Typically up to 48–72 hours; perfusion bioreactors may extend viability to 5–7 days.
Challenges and Considerations
- Heterogeneity: Tissue variability can affect reproducibility.
- Oxygen Diffusion: Thicker slices may experience central hypoxia.
- Standardization: Requires harmonized protocols across laboratories.
Future Directions
Innovations in organ-on-chip systems, 3D bioprinting, and multi-omics integration are driving PCLS toward applications in personalized hepatology and precision medicine.
Conclusion
Precision-Cut Liver Slices are a robust and physiologically relevant model for liver research. Mastery of vibratome parameters is critical to ensuring consistency and viability. With growing adoption in academic, clinical, and pharmaceutical settings, PCLS are set to play a pivotal role in the future of hepatology.
References
Leaker, B. D., Wang, Y., Tam, J., & Anderson, R. R. (2024). Analysis of culture and RNA isolation methods for precision-cut liver slices from cirrhotic rats. Scientific Reports, 14(1), 1–10. https://doi.org/10.1038/s41598-024-66235-2
Rodimova, S. A., Kozlov, D. S., Krylov, D. P., Mikhailova, L. v., Kozlova, V. A., Gavrina, A. I., Mozherov, Elagin, V. v., & Kuznetsova, D. S. (2024). Nanoparticles for Creating a Strategy to Stimulate Liver Regeneration. Sovremennye Tehnologii v Medicine, 16(3), 31–41. https://doi.org/10.17691/STM2024.16.3.04
Very, N., Boulet, C., Gheeraert, C., Berthier, A., Johanns, M., Bou Saleh, M., Guille, L., Bray, F., Strub, J. M., Bobowski-Gerard, M., Zummo, F. P., Vallez, E., Molendi-Coste, O., Woitrain, E., Cianférani, S., Montaigne, D., Ntandja-Wandji, L. C., Dubuquoy, L., Dubois-Chevalier, J., … Eeckhoute, J. (2024). O-GlcNAcylation controls pro-fibrotic transcriptional regulatory signaling in myofibroblasts. Cell Death & Disease 2024 15:6, 15(6), 1–22. https://doi.org/10.1038/s41419-024-06773-9
Wang, Y., Leaker, B., Qiao, G., Sojoodi, M., Eissa, I. R., Epstein, E. T., Eddy, J., Dimowo, O., Lauer, G. M., Qadan, M., Lanuti, M., Chung, R. T., Fuchs, B. C., & Tanabe, K. K. (2024). Precision-cut liver slices as an ex vivo model to evaluate antifibrotic therapies for liver fibrosis and cirrhosis. Hepatology Communications, 8(11). https://doi.org/10.1097/HC9.0000000000000558
Xu, M., Warner, C., Duan, X., Cheng, Z., Jeyarajan, A. J., Li, W., Wang, Y., Shao, T., Salloum, S., Chen, P. J., Yu, X., Chung, R. T., & Lin, W. (2024). HIV coinfection exacerbates HBV-induced liver fibrogenesis through a HIF-1α- and TGF-β1-dependent pathway. Journal of Hepatology, 80(6), 868–881. https://doi.org/10.1016/J.JHEP.2024.01.026
Upcoming Events
-
Oxford Neuroscience Symposium
Date: March 18, 2026 -
Cambridge Neuroscience Seminar
Date: April 1, 2026 -
Symposium of the Young Physiologists
Date: April 8, 2026