Why Adrenal Glands?
- Fully translational model: this application highlights the use or both murine and human adrenal glands
- Peculiar embryological origin: ectoderm-derived neuroendocrine medulla, secreting catecholamines, and a mesoderm-derived cortex
- Can be investigated using many methods: acute slice preparations provide a good access for experimental interventions while maintaining cellular interactions within the tissue
Challenges
- Small Tissue Size: careful work and extra time is required to obtain optimal conditions for slicing
- Hypoxia: all work is carried out in bicarbonate-buffered salt solution that are constantly gassed with carbogen to maintain oxygenation
- Difference between murine/human tissue samples: mice have a higher, strain-dependent osmolality of ~315 mOsmol while the osmolality of human plasma centers around 290 mOsmol
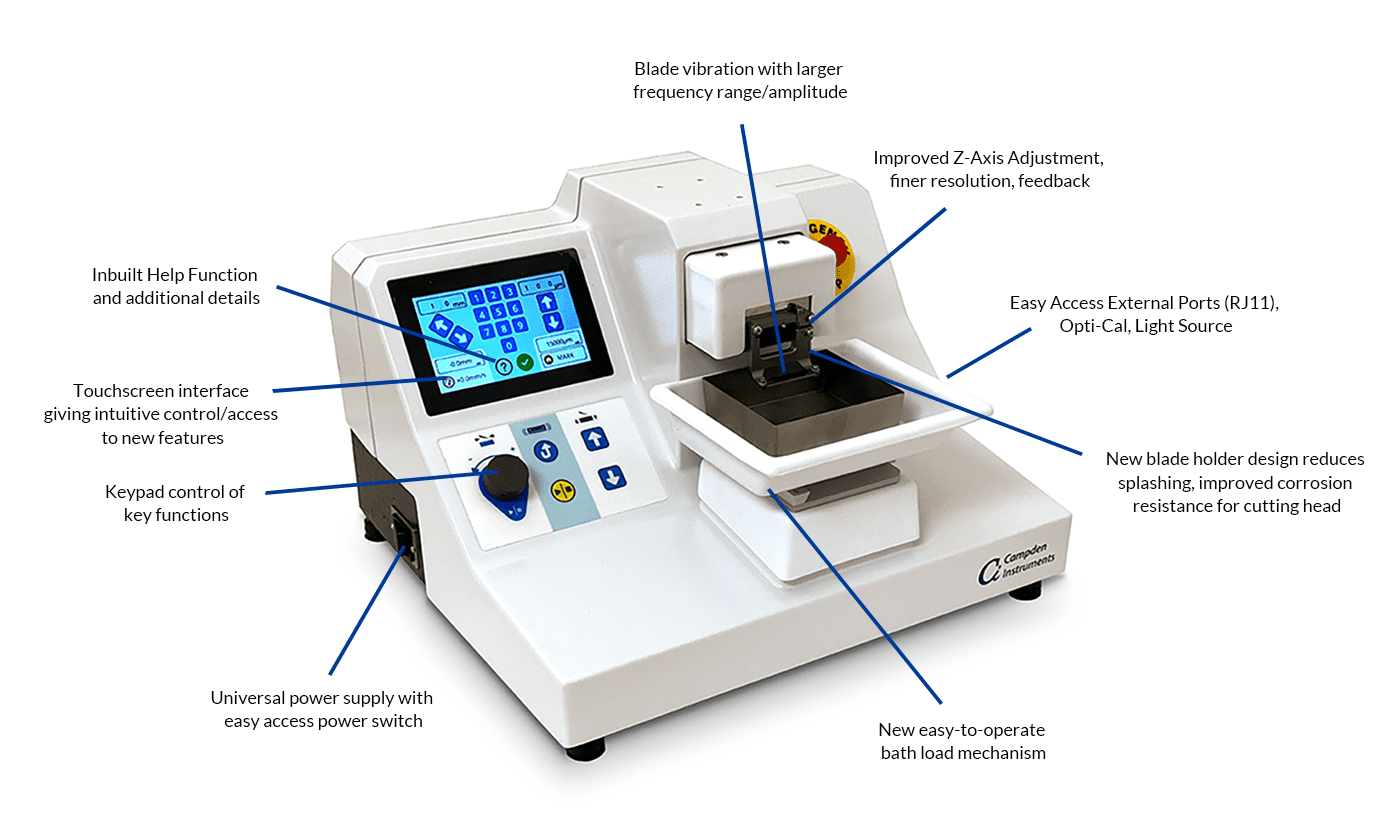
Process
Review the full Application note for detailed procedures and working examples.
- Clean Preparation of Adrenal Tissue including blotting for excess liquids
- Obtain 4% concentration low-melting temp agarose
- Place glands – if necessary, it is possible to embed both mouse adrenal glands into the same agarose well
- Mount agarose to vibratome stage and place on cutting tray filled with bicarbonate-buffered solution
- Begin slicing process
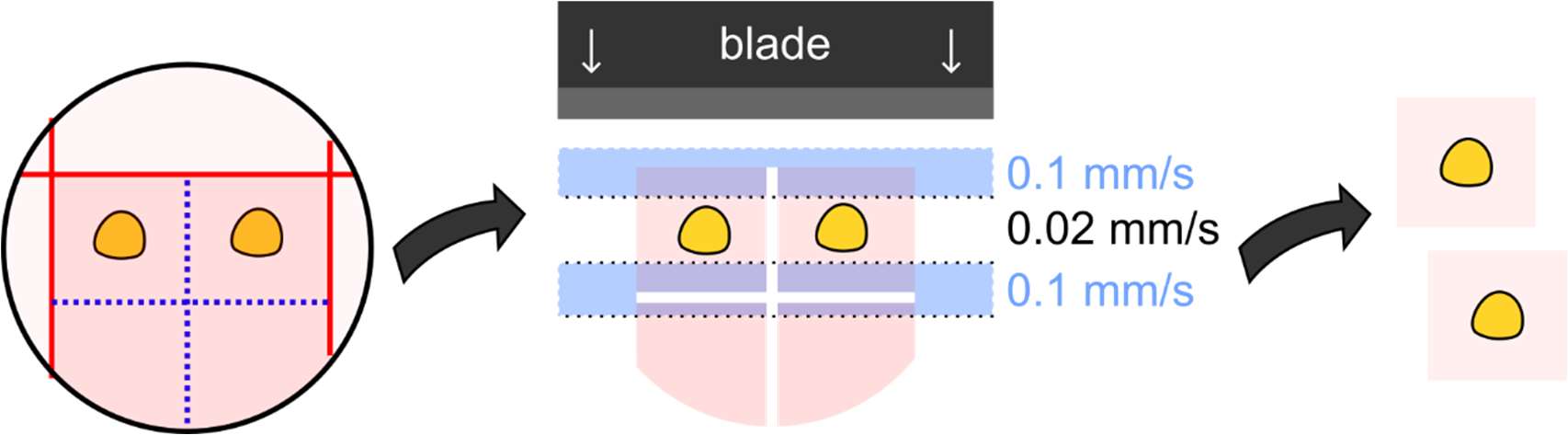
Figure 1: Schematic drawing of the steps required to generate acute murine adrenal slices.
| Parameter | Recommended Setting |
|---|---|
| Slice Thickness | Initially 500 µm, slowly reducing setting to the desired final thickness |
| Sectioning Speed | 0.1 mm/s through agarose, 0.02 mm/s through tissue |
| Vibration Frequency | 90 Hz |
| Amplitude | 1.5 mm |
| Temperature | ~37 °C |
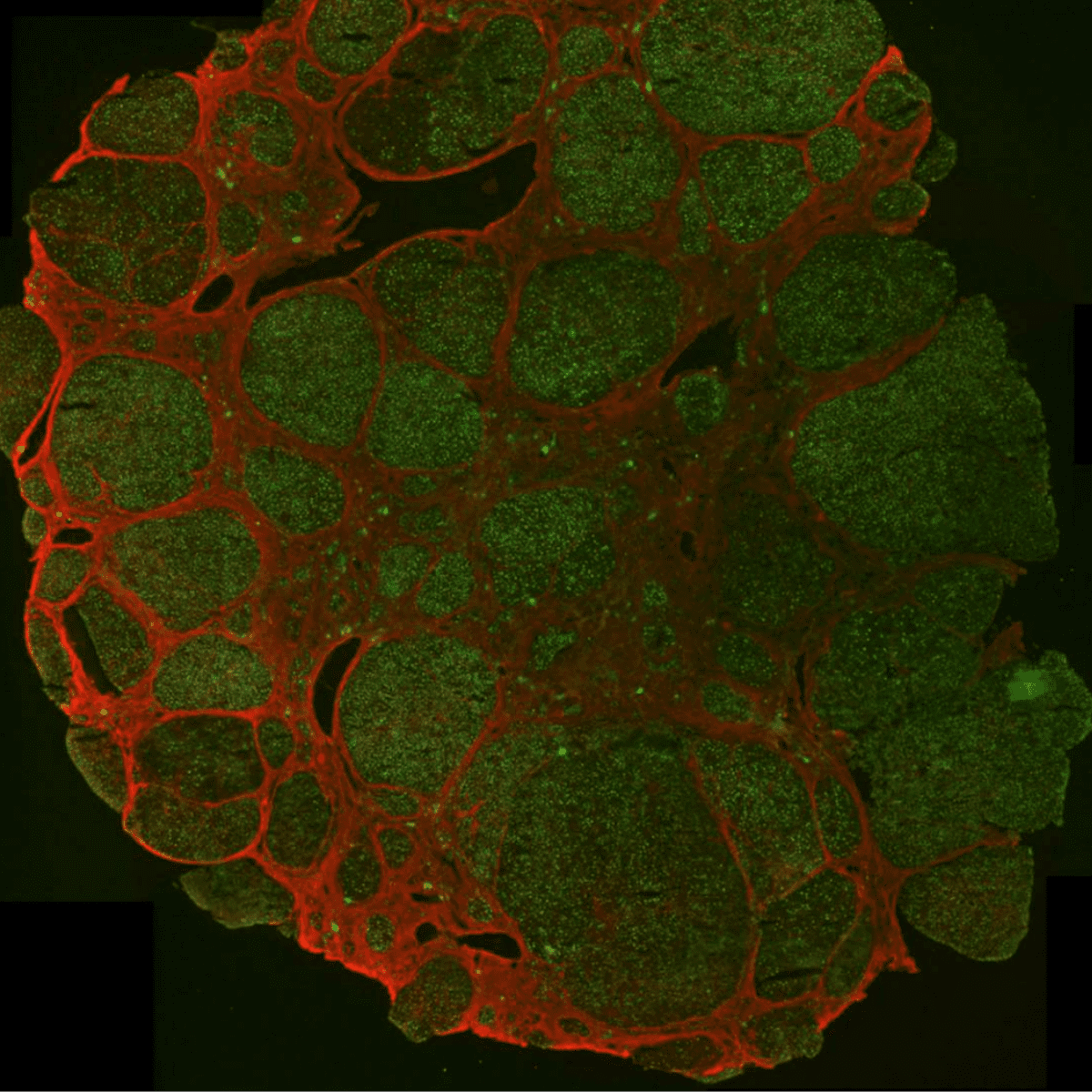
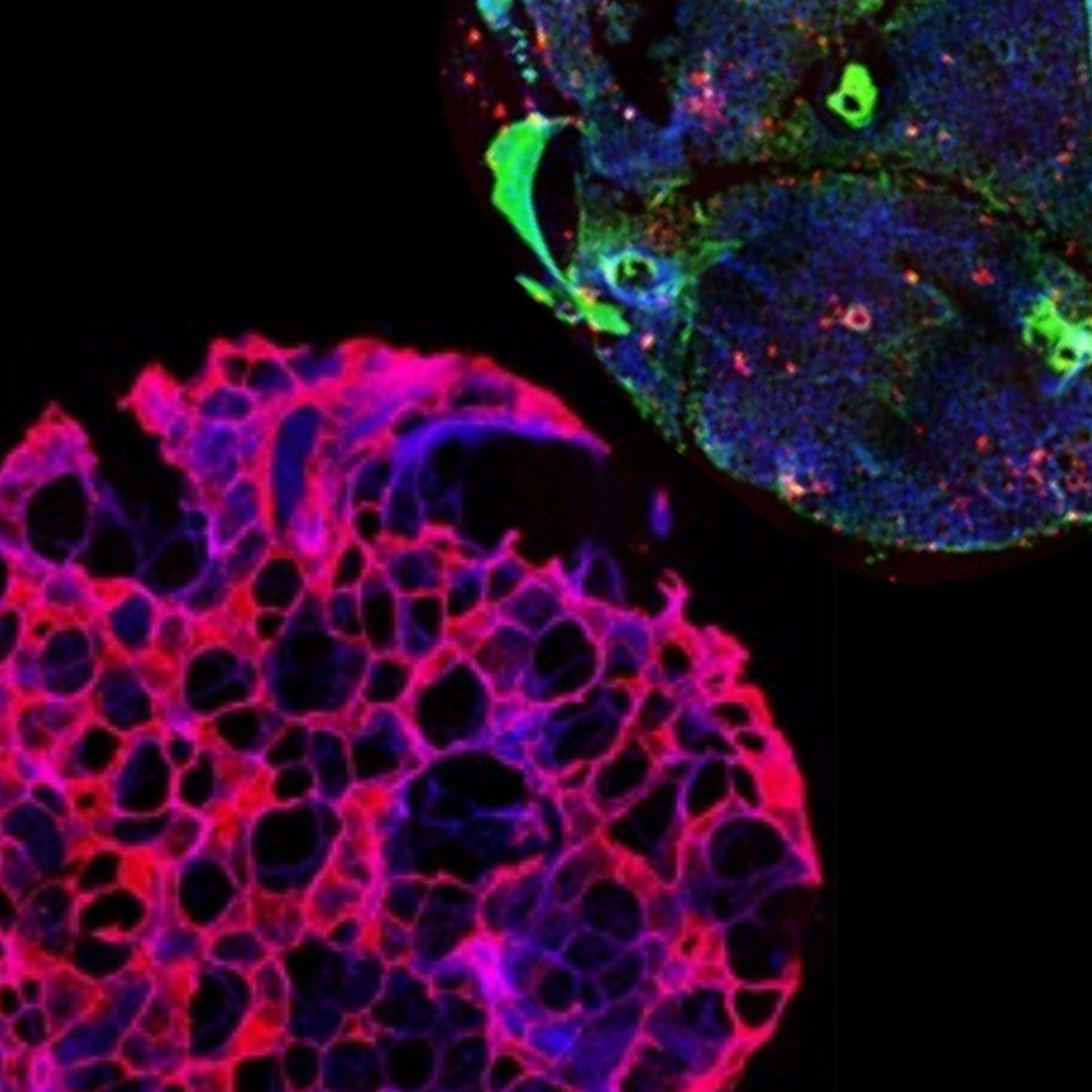
The adrenal cortex is rather opaque with thin slices (60-80 µm) having the best optical transmissibility allowing for easier identification of single cells, for example in patch clamp studies, at the expense of reducing yield. Medium slices (120-160 µm) are a good compromise of providing good yield, reasonable optical transmissibility for fluorescence experiments, in particular calcium imaging, and mechanical stability. Thicker slices (≥200 µm) are suited for long-term incubations, purification of nucleotides and proteins as well as observations of hormone production. They provide a low yield overall due to the small number of slices being generated from a single adrenal gland but excellent mechanical stability. For human samples, 200 µm thick slices are a more reasonable overall choice because of the larger organ size.
Following cutting, slices can be stored in carbogen gassed solutions for up to 8 hours before a drop in the quality and reproducibility of results becomes apparent. It is possible to maintain mouse adrenal tissue slices in an interface culture for at least one more day and human slices can be maintained for 3 days typical calcium transients still being observable. However, we have not made any systematic evaluation into whether these signals are fully comparable to those measured on the first day.
Selected References
Schewe, J.; Seidel, E.; Forslund, S.; Marko, L.; Peters, J.; Muller, D. N.; Fahlke, C.; Stelting, G.; Scholl, U. Elevated Aldosterone and Blood Pressure in a Mouse Model of Familial Hyperaldosteronism with CIC-2 Mutation. Nat Commun 2019, 10 (1 ), 5155. https://doi.org/10.1038/s41467-019-13033-4.
Seidel, E.; Schewe, J.; Zhang, J.; Dinh, H. A.; Forslund, S. K.; Marko, L.; Hellmig, N.; Peters, J.; Muller, D. N.; Lifton, R. P.; Nottoli, T.; Stelting, G.; Scholl, U. I. Enhanced Ca2+ Signaling, Mild Primary Aldosteronism, and Hypertension in a Familial Hyperaldosteronism Mouse Model (Cacna1hM1560V/+). Proceedings of the National Academy of Sciences 2021, 118 (17), e201487 6118. https://doi.org/10.1073/pnas.201487 6118.
Barbara, J.-G.; Christophe Poncer, J.; Anne McKinney, R.; Takeda, K. An Adrenal Slice Preparation for the Study of Chromaffin Cells and Their Cholinergic Innervation. Journal of Neuroscience Methods 1998, 80 (2), 181-189. https://doi.org/10.1016/S0165-0270(97)00200-8.
Barbara, J. G.; Takeda, K. Quantal Release at a Neuronal Nicotinic Synapse from Rat Adrenal Gland. Proc. Natl. Acad. Sci. U.S.A. 1996, 93 (18), 9905-9909. https://doi.org/10.1073/pnas. 93.18. 9905.
Dinh, H. A.; Volkert, M.; Secener, A. K.; Scholl, U. I.; Stelting, G. T- and L-Type Calcium Channels Maintain Calcium Oscillations in the Murine Zona Glomerulosa. Hypertension 2024, 81 (4), 811-822. https://doi.org/10.1161 /HYPERTENSIONAHA.123.21798.
Guagliardo, N. A.; Klein, P. M.; Gancayco, C. A.; Lu, A.; Leng, S.; Makarem, R. R.; Cho, C.; Rusin, C. G.; Breault, D. T.; Barrett, P. Q.; Beenhakker, M. P. Angiotensin II Induces Coordinated Calcium Bursts in Aldosterone-Producing Adrenal Rosettes. Nat Commun 2020, 11 (1), 1-15. https://doi.org/10/ghj45b.
Hu, C.; Rusin, C. G.; Tan, Z.; Guagliardo, N. A.; Barrett, P. Q. Zona Glomerulosa Cells of the Mouse Adrenal Cortex Are Intrinsic Electrical Oscillators. Journal of Clinical Investigation 2012, 122 (6), 2046-2053. https://doi.org/10/f32hq7.
Upcoming Events
-
Oxford Neuroscience Symposium
Date: March 18, 2026 -
Murine Muscle Mechanics Workshop
Date: April 29 - May 1, 2026 -
UCSF Mission Bay Vendor Show
Date: January 15, 2026